![]()
Equipment used:
radioactive sources: 207Bi, 137Cs, 22Na,
and 24Na; NaI(TI) crystal and PMT with associated electronics;
multichannel analyzer card in personal computer
Objectives:
Interaction Mechanisms
Gamma rays are electromagnetic radiation with wavelength l of less than about 1 nm = 10-9 m. This corresponds to an energy of greater than 1.6 x 10-16 Joule or 1 keV. Gamma rays are emitted when nuclei undergo transitions from higher-energy to lower-energy states.
There are three principal mechanisms by which gamma rays interact with matter: the photoelectric effect, Compton scattering, and pair production. Each is a consequence of the electromagnetic interaction.
(a) Photoelectric effect. You have already considered the photoelectric effect from the standpoint of ejection of electrons from a metal cathode. A minimum energy (work function) had to be provided to overcome the relatively weak attraction of a chunk of metal for an electron. Similarly an electron can be ejected from an atom by radiation with sufficient energy to overcome the binding of the electron to the atom. If the incident photon has energy Eg , the electron is ejected - usually from the innermost (K) electron shell - with kinetic energy Ee = Eg - B, where B = binding energy of the electron. The probability s of ejection of photoelectrons depends on both the photon energy Eg and the number (Z) of electrons per atom. It can be expressed approximately in terms of,
![]()
for low (<100 keV) photon energies. At higher energies, the probability of ejection varies more slowly with energy and varies with a slightly higher power of Z.
(b) Compton scattering. The g photon scatters from, and imparts energy to, an electron. By treating the scattering as a collision between two particles and writing down equations for conservation of total energy and the components of momentum (with photon energy Eg and momentum Eg /c), you can derive a number of relationships.
(1) The wavelength shift of a photon which has scattered through an angle q.
![]()
Note: The electron rest energy and Compton wavelength are found as follows.
![]()
h/(moc) = 2.43 x 10-12 m
(2) Thus, the energy acquired by the electron when it is struck by a photon is Ee.
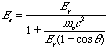
Note that there is a maximum value of the energy the electron
can acquire, namely,
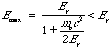
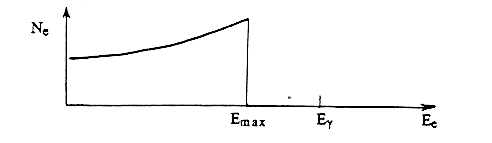
corresponding to the case in which the photon is "backscattered," scattered back in the direction from which it came. However, the energy acquired by the electron may be any value down to 0, depending on the angle q through which the photon scatters. The theoretical number of electrons as a function of their energy Ee is shown in Fig. 1.
(c) Pair production. If it has sufficient energy, a photon may be converted into an electron-positron pair. Clearly the minimum energy necessary equals the sum of the rest-energies of the electron and positron,
2 mo c2 = 2 x 0.511 MeV = 1.022 MeV.
(In this case it is easy to see why pair production must take place in the presence of a nucleus or electron; something has to absorb the photon's momentum Ey/c !) Any additional photon energy beyond this minimum goes into kinetic energy. The probability of pair production must be zero below 1.022 MeV; pair production becomes more likely than Compton scattering only for photon energies above several MeV.
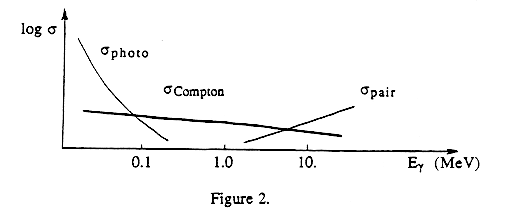
Figure 2 summarizes qualitatively the relative probabilities s of the three mechanisms as a function of photon energy.
DETECTION OF GAMMA RAYS
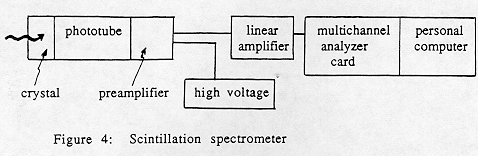
In this experiment, gamma rays are detected by a scintillation crystal made of thallium-activated sodium iodide, abbreviated NaI(TI). The energy deposited in the crystal is converted to a flash of light, whose intensity is proportional to the energy of the gamma ray. This is converted to a voltage pulse by a photomultiplier tube (PMT, for short) and associated electronics.
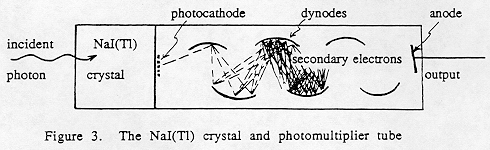
The detection process is as follows (Fig. 3):
2. The radiation strikes the cathode of the PMT and produces low energy electrons by means of the photoelectric effect.
3. High voltage applied to the PMT accelerates the photoelectrons down a series of electrodes ("dynodes"), where more electrons are produced by secondary emission.
4. The electron current at the final electrode (anode) is integrated, and an output voltage pulse is formed.
The amplitude of the output voltage pulse is proportional to the energy lost by the g -ray in the crystal. However, the pulse is only a fraction of a volt high. For analysis, it must be amplified while retaining the linear relationship between g ray energy and output voltage. The amplified voltage pulses are sorted by a multichannel analyzer, a "voltmeter with a memory" which records how many pulses in each voltage "channel" (hence g ray energy) were produced. This is the gamma ray spectrum.
GAMMA RAY SPECTRA
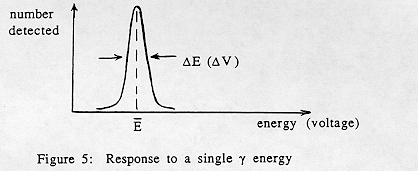
An idealized spectrometer would give you a "spike" of a single voltage corresponding to a single gamma ray energy deposited. In practice, electronic noise in the spectrometer, as well as statistical fluctuations in the process of converting photon energy to voltage, broaden the peak even when only a single gamma ray energy is deposited (Fig. 5). The narrowness of the experimental peak is a measure of the quality of the detector. We define the resolution of the detector as DE, where DE is the full width of the peak at half maximum (FWHM). Sometimes this is expressed as DE /E or (DE /E) x 100%. The significance of D E is this: if two gamma rays produce mean responses E1 and E2, they will be resolved if |E2 – El | > DE.
When a gamma ray of energy Eg strikes the scintillation crystal, so long as the full energy is collected within a microsecond, a voltage pulse corresponding to Eg results. This is so even though the collection process may be somewhat complicated. For example in the photoelectric effect, the ejected electron has energy Eg - B, which it gives up to the crystal primarily by colliding with electrons and exciting or ionizing the atoms. Photons, emitted as several atoms de-excite or capture electrons to fill vacancies (including that left by the
original photoelectron!), can be collected at the photocathode and their energies added, together corresponding to the full energy Eg . Similarly in Compton scattering, if the energies of both the electron and the scattered photon are collected within a microsecond, a voltage pulse corresponding to Ey results.
However, even when gamma rays of only a single energy enter the scintillation crystal, the full energy is not always collected, so the spectrum is more complicated than a single peak. Some features to look for include the following.
RADIATION SAFETY
The radioactive sources used in this experiment are only a few microcuries in strength, and they are all sealed, so they actually pose no significant health hazard. However we will follow good safety procedure. Horseplay involving the sources is forbidden. While the sources are not dangerous, like any radioactive source they should be treated with respect and properly handled. Only the instructor is to handle the sources, and he/she will wash hands after handling the sources. When any source is not in use, it is to be stored behind lead shielding. In the event of any accident which results in spilled radioactivity, do not attempt to clean it up yourself. Block off the area of the spill and call the professor in charge of the course.
Remember: If for safety reasons you choose not to work directly with radioactive sources, you are given the option of performing the experiment with stored data instead. See the instructor to arrange a time when you can do this.
USING THE GAMMA RAY SPECTROMETER
Do not turn off the power to the electronics, except for the personal computer and printers. The high voltage supply and amplifier may be safely left on all the time.
The size of the NaI(TI) crystal determines its efficiency in detecting gamma rays, and the attached phototube determines the high voltage and amplification required. You should note down the size of the NaI(TI) crystal provided at your laboratory station.
Detailed description of the use of the PCA-II software is given on a separate page.
The software allows you to calibrate each spectrum, so that arbitrary voltage units ("channels") are converted to photon energy. To calibrate, you specify the energy that corresponds to each of two or more full-energy peaks; to reduce extrapolation error these should be far apart. Because the peaks in the scintillation spectrum are broad, and because you are extrapolating (or interpolating) over a sizable energy range, your gamma ray energy values are only accurate to ±10 keV or ±20 keV, where 1 keV = 1.60 x 10-16 joules. (It is much easier to locate exactly the much narrower full-energy peaks in a spectrum recorded with a high-resolution germanium spectrometer, and so calibration is much more precise with such a detector.)
Gamma Ray Spectroscopy with the PCA-II Card
Again we use the PCA-II card and software, which you first used in the K-capture atomic spectroscopy experiment, to convert the personal computer into a multichannel analyzer. The software really is user-friendly; the commands on the pull-down menus mean about what you'd expect them to mean. Suggestions are provided below for initial choices of parameters. Try to refrain from exploring all the possibilities of the software so you do not get distracted from learning the physics.
(F#) denotes a Function Key. Toggle between the spectrum window and the command area by clicking the right mouse button.
I. Starting PCA Software
A. At the C:\PHY293> type mca and then enter (<-)II. Setting up to record a spectrum (Use the mouse to choose from the menu bar.)
A. Setup
4. No overlap (overlap).
III. Recording a Spectrum
B. You can erase a spectrum after [F1] - Stop by pressing Ctrl and [F2].
IV. Calibration
2. You can delete an ROI with [Del], and you can toggle on/off
the shading of the ROI with [Alt] and R.
2. Locate Peak 1 by putting the cursor within the ROI on Peak 1. <Enter>. Type in the energy for this peak.
3. Repeat for each calibration peak. When you have entered the last calibration peak, type <Enter> again, and you will be told that calibration is terminated.
FOR YOUR REPORT
You are to obtain and analyze gamma ray spectra as described below. You should print out each of the spectra. Write the results of your measurements directly on these hardcopy spectra.
Record another spectrum of 137Cs in which you deliberately reduce the backscatter peak by removing the lead brick behind the source, with everything else unchanged. When you do this, one peak disappears from the spectrum in the vicinity of 100 keV. Identify what it is, and explain why it arises only when the lead brick is put in place.
208TI: This isotope, historically called Th C", is part of
the decay chain of Th-232, which has a half life of 14 billion years. It
emits gamma rays of 511, 583, 860, and 2614 keV.
| This document was last modified on Tuesday, 07-Nov-2000 17:27:08 EST
and has been accessed [an error occurred while processing this directive] times. Address
comments and questions to:
|