 |
The name zircon originated from the Persian zar, meaning gold and gun, meaning color, referring to the golden orange-brown color of some varieties of zircon.
Zircon is one of the oldest minerals found in the earth's crust. It is a common accessory mineral occurring in low concentrations in igneous and metamorphic rocks, as well as an abundant mineral in sedimentary rocks and in alluvial deposits in beach sands. Since zircon is one of the earliest minerals to solidify it is always crystalline in acid eruptive rocks. It is often enclosed in later minerals but may form large, well-developed individual crystals in granite and pegmatites. The size and morphological character of zircon, particularly the length to width ratio, may be closely similar throughout a body of magmatic granite. It has been suggested that the absence of such a relationship may indicate that an intrusion is complex. Important commercial deposits are mined in Australia, India, South Africa, and the USA Some of the more important or interesting localities for zircon include:
Russia, Ilmen Mountains: crystals up to 3.5kg of prismatic habit occur in veins in granite associated with orthoclase, mica and apatite and in the Ural Mountains where crystals up to 5cm have been found.
Canada, Renfrew County, Ontario: crystals up to one foot long and weighing 7kg have been observed.
Brazil, Minas Gerais: has produced light-green transparent crystals. Germany, Eifel Mountains: as small druses of colorless zircons in trachyte cavities.
Austria, Tyrol region: zircon has been found associated with pink titanite and chlorite.
Thailand, Cambodia, South Vietnam: are the main producers of gem zircon. It occurs as water-worn pebbles in gem gravels at depths usually less than 10 feet.
Australia: zircon is extracted from sands. The most important source is beach deposits on the coast of Queensland. These produce 250,000 tons annually.
[Top]
Zircon, or zirconium silicate (ZrSiO4) belongs to the neosilicate class of silicate minerals. Neosilicate ("island" silicates) are so called because they consist of isolated SiO4 tetrahedra. Each zirconium atom is located between 8 oxygen atoms arranged at the corners of a triangular ZrO8 dodecahedron.
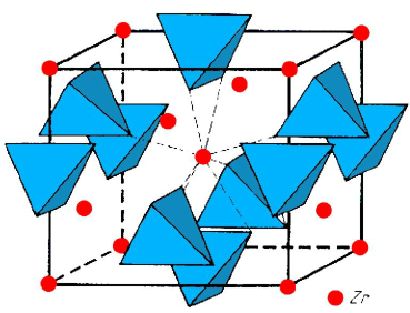
The basic structural unit is therefore a chain of alternating edge-sharing SiO4 tetrahedra and ZrO8 dodecahedra.
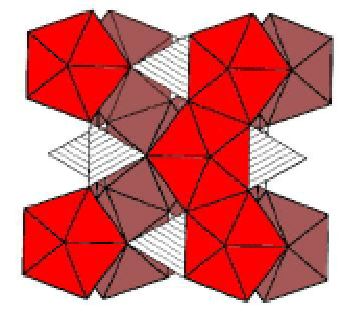
[Top]
Zircon always contains some Hf (usually 1-4 at%) and may also contain trace amounts of U and Th, which is why most zircon minerals are in a more or less amorphous state, the so-called metamict state, owing to radiation damage from a-decay of these impurities. Over the course of hundreds of millions of years, a-decay doses as high as 1019 decays/g can occur, which may lead to the complete amorphization of the zircon structure. Depending on the degree of damage, physical and chemical properties of metamict zircon can be quite different from those of crystalline zircon. Amorphization of zircon has significant consequences for the radioactive U/Pb dating method, as the parent/daughter isotope systematics are distorted. Metamict zircon can, in most cases, be converted to crystalline zircon by heating to between 1000 and 1450oC.
[Top]
The presence of U and Th makes zircon one of the most popular minerals for radiometric U/Pb age dating of geologic specimens as old as two billion years. Besides being of interest to the geochronology community, zircon is also of considerable technological importance. Because of its low thermal expansion coefficients and high thermal shock resistance, zircon finds widespread use in the ceramic foundry and refractory industries. Zircon is the primary mineral resource for the production of zirconium metal (Zr) and zirconia (ZrO2). Zirconia is one of the most refractory substances known and is the basis of a variety of advanced ceramic materials ranging from high ionic conductivity to high mechanical strength and toughness. Zirconium metal is hard and is used in the construction of nuclear reactors due to its low neutron-absorption cross-section, retention of strength at high temperatures, and excellent resistance to corrosion. Zirconium is also alloyed with aluminium, manganese, silicon and nickel to produce hard alloys. Zircon has been shown to be a potential optical wave-guide material, and the zircon structure type is under study as a model substance for the immobilization of actinide-bearing radioactive waste.
[Top]
|
