![]()
from Dr. Paul J. Bottino
![]()
In order to identify transformed cells or plants that have been growing on a selective medium, it is necessary to have an easily assayable reporter gene. The most useful reporter genes encode an enzyme activity not found in the organism being studied. A number of genes currently are being used, however one of the most popular is the E. coli ß glucuronidase. The protein has a molecular weight of 68,200 and appears to function as a tetramer. It is very stable, and will tolerate many detergents, widely varying ionic conditions, and general abuse. It is most active in the presence of thiol reducing agents such as ß mercaptoethanol or DTT. It may be assayed at any physiological pH, with an optimum between 5.2 and 8.0. The GUS gene is usually used in a gene fusion. This means that the GUS coding sequence is under the direction of the controlling sequence of another gene. For this exercise the GUS gene is under the control of the Cauliflower Mosaic Virus 35S promotor.
The GUS gene was developed initially as a gene fusion marker in E. coli and in the nematode C. elegans, but has more recently been used extensively to monitor chimeric gene expression in plants. There is little or no detectable b-glucuronidase activity of yeast, Drosophila, C. elegans, Dictyostelium, or in almost any higher plant. Agrobacterium containing some of the GUS plasmids show significant GUS activity. This seems to be due to in part read-through transcription from the gene into which the GUS coding region might be located. Agrobacterium without these constructs shows little if any detectable GUS activity. In order to solve this problem, one laboratory has constructed GUS genes carrying an intron, which much be processed before expression takes place. This totally eliminates expression in any untransformed system.
The best substrate currently available for histochemical localization of b-glucuronidase activity in tissues and cells is 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc). The substrate works very well, giving a blue precipitate at the site of enzyme activity. There are numerous variables that affect the quality of the histochemical localization, including all aspects of tissue preparation and fixation as well as the reaction itself.
It is necessary to understand the nature of the reaction to better eliminate the variables. The product of glucuronidase action on X-Gluc is not colored. Instead, the indoxyl derivative produced must undergo an oxidative dimerization to form the insoluble and highly colored indigo dye. This dimerization is stimulated by atmospheric oxygen, and can be enhanced by using an oxidation catalyst such as a K+ ferricyanide/ferrocyanide mixture. Without a catalyst, the results are often very good, but one must be concerned about the possibility that localized peroxidases may enhance the apparent localization of glucuronidase.
Fixation conditions will vary with the tissue, and its permeability to the fixative. Glutaraldehyde which can be used, does not easily penetrate leaf cuticle, but is immediately available to stem cross sections. Formaldehyde seems to be a more gentle fixative than glutaraldehyde, and can be used for longer times.
Whole tissues, callus, suspension culture cells and protoplasts or whole plants or plant organs, can be stained, but survival of the stained cells is not by means certain. After staining, clearing the tissue with 70% ethanol seems to improve contrast in many cases.
Procedure
Tissue Fixative if fresh tissues are not used: (0.3% Formaldehyde, 10 mM MES pH 5.6, 0.3 M mannitol)
Store in fume hood at room temperature for 3 months, or until precipitates appear.
0.5 M MES 5.6:
For 100 ml, dissolve 9.76 g of MES in 80 ml of dH20. Adjust pH to 5.6 with NaOH and make to volume. Store at room temperature.
200 mM NaPO4 pH 7.0:
This is made by mixing two stock solutions. Stock A: 0.2 NaH2PO4 H20 (31.2 g/l).
Stock B: 0.2 Na2HP04 (28.39 g/l). For pH 7.0, Combine 39 ml A with 61 ml B.
X-GLUC Stain:
Dissolve 5 mg X-gluc in 1.0 ml dimethyl formamide. Add 50 mM NaPO4 pH 7.0 to 10 ml.
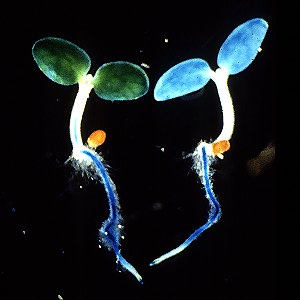
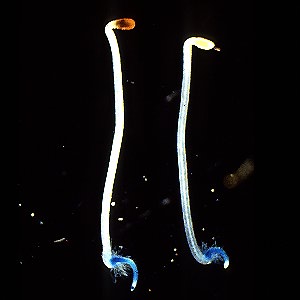
Left, light grown, right, dark grown tobacco seedlings expressing the GUS gene driven by the PAL1 promotor. Work of Dr. Tomoko Fugisaka Akada
Although spectrophotometric substrates for GUS are available, GUS activity in solution is usually measured with the fluorometric substrate 4-methylumbelliferyl-b-D-glucuronide (MUG). Fluorometry is preferred over spectrophotometry because of its greatly increased sensitivity and wide dynamic range. The assay is highly reliable and simple to use. Occasionally, endogenous compounds will interfere with the assay, either by quenching or by producing a high background fluorescence. In these situations, fluorometric substrates with differing excitation and emission wavelengths are available (the most popular is resorufin b-D-glucuronic acid). The substrate 4-trifluoromethylumbelliferyl b-D-glucuronic acid (4-TFMUG) allows continuous monitoring of GUS activity because, unlike MUG, it becomes fluorescent upon hydrolysis at the assay pH. In contrast, after hydrolysis of MUG by GUS, the reaction first must be terminated with basic solution. This not only stops the enzyme reaction, but also causes the fluoresc.
Procedure
Extracts can be stored at 70 C with no loss of activity for a long time, or at 4 C, with little loss of activity. Avoid storage at 20 C, which kills the enzyme in lysis buffer. If the extract is high in endogenous fluorescent compounds or that produce high levels of polyphenolics can be extracted in GUS extraction buffer with polyclar (insoluble polyvinyl pyrollidone) followed by a brief spin column of Sephadex G 25 to eliminate almost all polyphenolics and low molecular weight fluorescent contaminants from the extract.
Assay Buffer: 1mM MUG in extraction buffer
Dissolve 22 mg 4 Methyl umbelliferyl B D glucuronide (MUG) in 50 ml GUS extraction buffer in a 50 ml disposable polypropylene tube. Store at 4 C for up to two weeks.
Stop Buffer: 0.2 M Na2CO3 (1 liter)
Dissolve 21.2 grams of Na2CO3 in deionized distilled water. Make up to 1 liter.

REFERENCES