Manipulation of transfer cells to improve grain filling

| Introduction
|

| Partners
|
![]()
Introduction
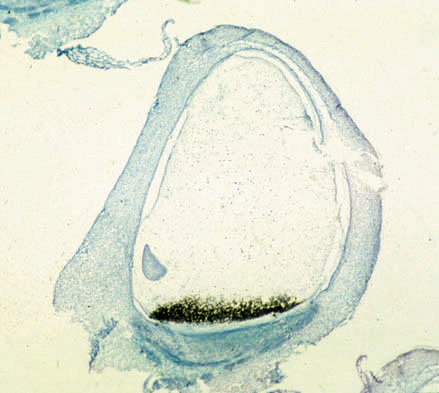
The transfer cell systemAgronomic selection in seed crops during domestication has usually resulted in many-fold increase s in seed weight. This has been achieved by corresponding increases in solute transfer from the plant body during seed development. The seed lacks symplastic connections with the maternal tissue layers, and to improve the efficiency of nutrient uptake by the developing seed, certain cell layers have become adapted for the transfer of water and solutes. In cereals these transfer cells are located in the basal endosperm, while in dicotyledons they occur in the epidermis of the cotyledon and in the seed coat. Transfer cells thus represent a seed-specific tissue, highly adapted for the transport of solutes, which play a pivotal role in determining the quality and quantity of the seed reserves. Marker genes for the maize basal endosperm transfer layer (BETL-1 and BETL-2; see Figure 1 ) have been recently characterized, and this project investigates the possibility of modifying, through the specific expression of transgenes, the transport function of these cells with regard to both the selectivity and efficiency of partitioning.
![]()
Partners
Five European laboratories have formed a network to focus efforts on transfer cell research. The participating labs., and their roles in the consortium, are listed below:
- Dr. R.D. Thompson, Max-Planck- Institut für Züchtungsforschung, Köln, D. Control of gene expression in transfer cells
- Dr. Giampiero Cai, Università di Siena, Siena. Subcellular structure and dynamics of transfer cells
- Prof. H.G. Dickinson, University of Oxford, Oxford, GB. Role of imprinting in regulation of endosperm development
- Dr. G. Hueros, Universita de Alcalà, Alcalà de Henares, ES. Identifying functions of transfer cell-specific proteins
- Dr. G. Donn, AgrEvo, Frankfurt, D. Maize transformat ion, biotechnological applications (sub-contract with Dr. Marion Kwart, MPI for Molecular Plant Physiology, Golm)
PARTNER 1: MPI for Plant Breeding Research, Cologne
Control of Gene Expression in Transfer Cells
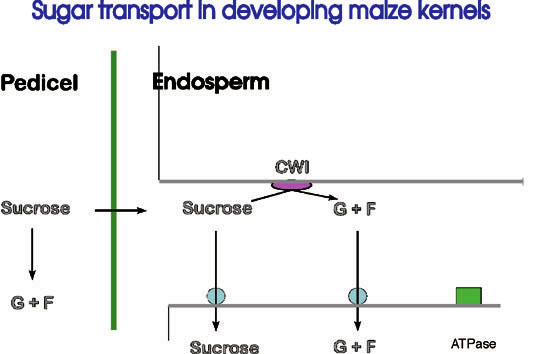
The Cologne group is interested in understanding the basis for transfer cell-specific expression. This will be investigated by fusing putative transfer cell-specific promoters to rep orter genes such as ß- glucuronidase (GUS), and by studying their expression in transgenic maize and tobacco. Once such a promoter is shown to be transfer cell- specific, it can be used to drive the expression of other genes of interest in transfe r layers. We are particularly interested in genes involved in carbohydrate assimilation in the endosperm which can be predicted to have major effects on grain filling and yield (Figure 2).
Figure 1: In situ hybridization of BETL-1 RNA. The mRNA, which is stained black, is located exclusively in endosperm transfer cells in this maize kernel section (16 days after fertilization, mi crograph prepared by Dr. S. Varotto, Univ. Padova).
![]()
PARTNER 2:
Biologia Ambientale, Univ. of Siena
Subcellular Structure and Dynamics of Transfer Cells
The Siena lab's contribution to the Transfer Cell Project will mainly focus on the ultrastructural analysis of developing transfer cells, accomplished by immunocytochemical observations. At the outset, a detailed electron microscopy analysis of developing transfer cells will be carried out to standardize methods of fixation and antigen preservation .
A second task of the Siena Unit will involve the production of monoclonal antibodies to cell wall proteins. The aim is to identify specific markers of the transfer cell differentiation process. The development of transfer cells will be monitored by using various different antibodies. In addition to novel antibodies raised in the course of the project, antibodies to cytoskeletal proteins (tubulins, centrosomal components, actin) and against arabinogalactan proteins (AGPs) will be investigated .
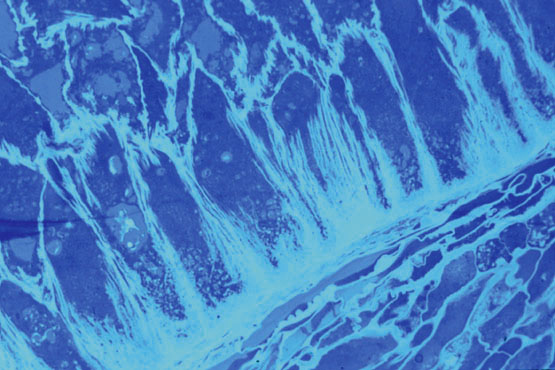
Figure 3: Cell wall structure of endosperm transfer cells Staining with a dye specific for secondary cell walls reveals the exist ence of arrays of lamellae attached mainly to the basal wall of each endosperm cell.(630x, oil immersion, photo courtesy of Ms M. Maitz, MPIZ).
![]()
PARTNER 3:
Plant Sciences, Univ. of Oxford
Role of Imprinting in the Regulation of Endosperm Development
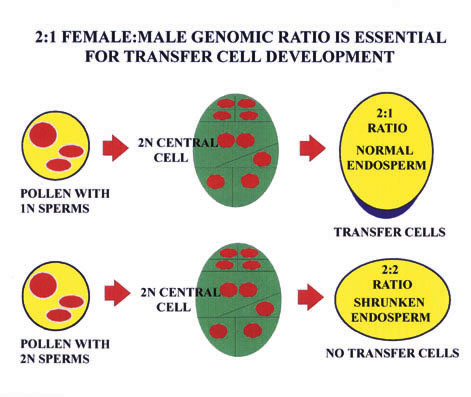
Ther e is strong circumstantial evidence that some aspects of transfer cell development are regulated by imprinted genes, for controlled pollinations with diploid pollen from tetraploid plants results in shrunken endosperms - and complete absence of any tra nsfer cell development (Charlton et al (1995) Development 121, 3089-3097.) [See Figure]. These pollinations result in an endosperm genomic ratio of 2x maternal to 2x paternal (rather than 2:1), suggesting that an imprinting system is "sensing" this ge nomic imbalance, and inducing aberrant development. In addition to determining whether any BETL genes are imprinted, The Oxford group will be exploring the possibility that known imprinted sequences - or those emerging from current screens - are expre ssed during transfer cell development.
![]()
PARTNER 4:
Biology Dept., Univ. Alcalà de Henares
Identifying Functions of Transfer Cell-specific Proteins
A series of BETL-specific clones has been isolate d and sequenced, however, much remains to be understood about their function. Four genes encoded small secreted proteins, with limited or no homology to other database accessions. Some possibilities for their function might be: modification of the cel l wall or plasma membrane to promote solute transfer (Figure 3), key metabolic steps to facilitate uptake of sugars and amino acids from the pedicel (Figure 2), protection against pathogen ingress. The Alcalà group is concerned with determining the properties of different groups of BETL-specific proteins, and are carrying out protein purifications and bioassays.
Figure 2: Pathway of sugar deposition into developin g seeds. Sucrose is unloaded from phloem terminals in the pedicel tissue and enters the endosperm apoplastically. A variable fraction (depending on the cereal species, for example) is hydrolysed by invertase to the monosaccharides glucose and fructose before import into the cell via specific H+ co- transporters. The process is energized by proton extrusion from the cell by membrane-bound ATPases.
![]()
PARTNER 5:
AgrEvo, Frankfurt-Höchst
Maize Transformation, Biotechnological Appl ications
One of the goals of this project is to generate maize plants possessing modified transfer cells. The AgrEvo group will be carrying out maize transformation, using PEG-mediated uptake into protoplasts derived from a unique and highly r egenerable cell line. Transformed calli are identified by phosphoinotricin selection. This system allows the production of a large number of stably transformed fertile maize lines for subsequent analysis of quantitative traits. In collaboration with Dr . Marion Kwart, (MPI for Molecular Plant Physiology, Golm), constructs for over-expressing invertase in the basal endosperm layer will be introduced into maize and tested for their effect on grain filling.

Figure 4: PAT-reisistance used to identify transformed maize plants The effectiveness of PAT (phosphoinotricin) selection is demonstrated by spraying a transformant and an untransformed control with PAT. (Photo courtesy of Prof. H.-H. Steinbiß, MPIZ)
![]()
Further information about this project can be found
here
![]()
![]() This webpage was produced by Ellen Peerenboom. The text was
provided
by Richard Thompson
This webpage was produced by Ellen Peerenboom. The text was
provided
by Richard Thompson