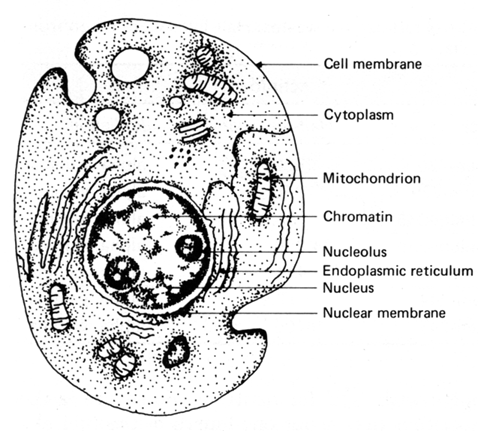
FIGURE 1: Schematic depiction of a cell showing its main components.
A Summary
In order to study small things like atoms and nuclei, we must use probes that have high energy, such as the emissions of radioactive sources. There is a certain amount of risk to health associated with use of radioactive sources. We want you to know that we are taking measures to protect your health. You should read this summary as well as the following section on Biological Damage due to Radiation. If, after considering the health risk in context, you do not wish to work directly with radioactivity, you may choose to perform the lab experiments using pre-recorded data.
Causes for concern are:
• cell death - replaced naturally unless exposure is extremely greatExtensive studies by the National Academy of Sciences Committee on Biological Effects of Ionizing Radiation suggest that even extremely low-level exposure to radiation produces a proportional, extremely low risk of cancer.
• cell damage - usually repairable, but the concern is for mutation and
• reproduction in undesirable forms - cancer.
You take many risks in ordinary living; in the US about 50,000 people per year die in automobile accidents. It does seem that the word "cancer" strikes particular fear, though today the disease is commonly treated successfully. Don't forget that newsmagazines are filled with reports of cancer risks from many sources, including smoking and many kinds of foods. How much radiation exposure should be cause for concern? The Environmental Protection Agency has concluded that the maximum allowable average exposure for the general public is 170 mrem/year above background. (A rem is a unit measuring energy deposited and effectiveness in damaging human tissue.) Background exposure, which results from cosmic rays and radioactive materials in your surroundings and in your own body, averages something like 190 mrem/year, resulting in a total allowable exposure of about 360 mrem/year. In the lab we'll use a survey meter that will demonstrate your exposure is a tiny fraction of that allowed by the E.P.A.
The essence of radiation protection consists of
• Time Minimize time of exposure to radiation.At Miami you may be exposed to sources which emit alphas (He nuclei), betas (electrons or positrons), or X-rays or gamma rays (high-energy photons).• Distance For a source emitting in all directions, doubling the distance to the source cuts the exposure to 1/4.
• Shielding Matter between you and the source absorbs or attenuates radiation. The sources you study will be shielded while being stored and counted.
In passing through matter, charged particles give up energy in ionization of the atoms. Electrons may undergo significant energy loss in elastic scattering from nuclei and as radiation emitted when they are slowed down (bremsstrahlung). Strongly interacting panicles may lose energy in interacting with nuclei. If enough matter is present, a charged panicle loses all its kinetic energy and is stopped; the distance required is called the range of the particle. A 1 MeV alpha has a range of about 1/3 cm of air, and a 1 MeV beta has a range of about 4 m in air or 2 mm in glass.
High-energy photons give up energy in matter by the photoelectric effect,
Compton scattering, and/or (for photon energy > 2 mc2)
production of electron-positron pairs. When a photon gives up all its kinetic
energy it ceases to exist; it is absorbed. The intensity of a beam of photons
entering a chunk of matter falls off exponentially with increasing thickness
of the absorber. A beam of 1 MeV photons is reduced to 1/e of its original
intensity by about 2 cm of lead.
Biological Damage due to Radiation
Fundamental Mechanisms for Biological Damage
Many of our discussions used the notion of a bound system built from some basic constituents. Human tissue can be viewed within this same framework with cells as the basic units (Fig. 1). Cells are responsible for both metabolic processes (movement, respiration, growth, and reaction to environmental changes) and reproduction. Cells controlling metabolism are termed somatic; those controlling reproduction are termed genetic. Control of these cell functions is exercised by the nucleus of the cell. Cells reproduce in tissue through a process of division called mitosis. The hereditary aspects of life are passed on from the parent cell to the divided ones (daughters) in chromosomes that are rich in a substance called deoxyribonucleic acid (DNA). The growth of a cell and its reproduction are governed by biochemical reactions involving many complex molecules. Damage to the molecules involved in these processes may alter the function of a cell to the extent that
2. it is damaged so that it cannot reproduce and therefore it eventually dies, or
3. it is still able to divide but the functioning of the new cells
is altered (mutated).

FIGURE 1: Schematic depiction of a cell showing its main components.
Some mutations may be inconsequential. However, some can produce somatic effects such as uncontrolled cell reproduction resulting in cancerous growths. Mutations in genetic cells can be passed on to succeeding generations.
Any mechanism that can supply the energy necessary to break a chemical bond. ionize an atom. or alter the chemistry of cells is capable of producing biological damage. Roughly it takes between 1 – 30 electron volts to damage a molecule. The alpha, beta, gamma, and neutron radiation from radioactive nuclei typically have energies in the range of millions of electron volts. Thus they are capable of damaging hundreds of thousands of cells. The actual damage produced depends on the physics of the interaction that, in turn, depends on the type and energy of the particle involved.
A particle loses energy by forces acting on it through some distance (work). The energy the particle loses is transformed (or converted) to other forms. For example, the brakes of an automobile exert a force on the wheels that decelerates the automobile. The kinetic energy of the car is converted into heat. A charged particle, like a beta or an alpha particle, loses its energy primarily through the electric interaction with the charged particles associated with atoms. Atoms may absorb energy and be excited to higher energy states or, if enough energy is transferred. electrons may be completely removed from the atoms. The rate of energy loss by a particle depends on the energy, charge, and mass of the radioactive particle. An alpha particle, being about 7000 times as massive and having twice the charge of an electron. loses energy at a much greater rate. For example, a one million electron volt alpha particle cannot penetrate a sheet of paper. But it takes many sheets of paper to stop a one million electron volt beta particle. Because an alpha particle deposits most of its energy in a small region of space, it produces much more local damage than a beta particle of the same energy.
A neutron, by virtue of having no charge, is very difficult to stop. It loses energy by collisions with nuclei the same way billiard balls lose energy in collisions. If a neutron makes a collision and loses its energy, it can do considerable damage. Because a neutron does not lose energy continuously as does a charged particle, it is not meaningful to talk about a thickness required to stop a neutron of given energy. Rather one talks about a thickness required to reduce the intensity of a neutron source by some given amount. Concrete is often used as a material to attenuate neutrons. It takes ten inches of concrete to reduce the intensity of a 10 million electron volt neutron source by 90%.
A gamma ray has no mass and no charge, but it does have electromagnetic energy and experiences electromagnetic forces when traversing matter. A gamma ray loses energy by three mechanisms:
2. It may be absorbed by an atom whereupon energy is relinquished to one of the electrons surrounding the nucleus. This is called the photoelectric effect.
3. It may interact with an atom and create an electron (e-)
and a positron (e+). This is called pair production.
Pair production requires a minimum of 1.022 million electron volts of energy
to compensate for the masses of the electron and positron.
Radiation Units
A nine-ounce baseball and a nine-ounce chunk of glass moving 60 miles per hour have the same kinetic energies. To a physician, who has to repair the damage inflicted on a person having the misfortune to intercept these particular objects, they produce quite different effects. The situation for nuclear radiation is quite similar. Different effects are produced by different types of radiation.
For some situations, we need to know only how much energy is deposited in some type of matter. This is referred to as a dose. The earliest unit for quantifying this concept was the Roentgen (abbreviated R). It is a useful measure for X-rays and gamma rays but is not a unit appropriate for radiation in general. A more appropriate unit is the rad, meaning radiation absorbed dose. One rad is defined as 0.00001 joules of energy absorbed per gram of substance. When the substance is human tissue, the rad and Roentgen are essentially the same and the terms are often used interchangeably. The rem, meaning roentgen equivalent man, is a biological unit accounting for the biological damage produced. It is much less precise than a physical unit like a rad because of the many factors entering into biological damage. The energy of the particle is a major consideration. For example, both visible light and gamma rays are electromagnetic radiation. However, a photon of visible light lacks a sufficient amount of energy to be biologically dangerous. Intense local tissue damage can be more serious than diffuse damage. For this reason, an energetic fission fragment that travels a very short distance in tissue is more dangerous than 250-keV X-ray photons, that distribute their energy over a much longer distance. Also, some body organs are much more susceptible to radiation damage than others. Because the amount of radiation damage is directly proportional to the dose received, the rem and rad are related to each other. But, because some radiation is more effective than others in producing biological damage, the proportionality factor, called the relative biological effectiveness (RBE), depends on the type and energy of the particle. Stated formally, dose equivalent in rems = RBE times dose in rads.
DE = RBE . D.
It is customary to use the damage
done by a whole body irradiation of 250-keV X-rays as the norm and assign
a RBE of unity to this radiation. The RBE for any other particle is then
measured relative to this standard. Table 1 lists some representative RBEs
for various radiations of interest.
Background Radiation
Knowing that radiation can produce very undesirable biological damage, it is only natural to seek protection by avoiding radiation exposure. It is, however, impossible to avoid all radiation because everyone is routinely exposed to a variety of natural and manufactured radiation sources. As inhabitants of the earth we are continually bombarded with nuclear radiation coining from unstable nuclei in the earth (Table 2) and from radiation produced by the interaction of cosmic rays with elements of the atmosphere. Cosmic rays are primarily very high-energy protons and gamma rays of extraterrestrial origin. There is considerable variation in the intensities of these radiations depending on geographic location and altitude. The cosmic background radiation at Boulder, Colorado, may be a factor of two larger than it is in an eastern city that is at a substantially lower elevation. The water from wells in Maine has some 3000 times more radium content than water from the Potomac River. On the average, each person in the United States receives an annual exposure of 50 mrem from cosmic radiation and 50 mrem from the earth and building materials. An additional 25 mrem is received internally from inhalation of air (5 mrem) and isotopes formed naturally in human tissue (20 mrem). From diagnostic X-rays and radiotherapy, a person receives about 60 mrem and from the nuclear industry, television, radioactive fallout, etc., 5 mrem. Thus the grand total comes to about 190 mrem/year.
TABLE 1: Some representative RBEs.
|
|
|
|
|
|
hematopoietic system critical |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TABLE 2: Primary
radionuclides from the earth that contribute to background radiation.
|
|
|
|
|
(226Ra) |
|
|
|
(238U) |
|
|
|
(232Th) |
|
|
|
(40K) |
|
|
|
(3H) |
|
|
|
(14C) |
|
|
Radiation Doses for Various Somatic Effects
Human beings will always be exposed to some uncontrolled radiation. Some risk will always be taken when there is exposure to additional amounts. Naturally, we want to know how much radiation is required to produce a given effect. This is a very difficult question to answer because
2. damage to genetic cells shows up only in succeeding generations.
Nuclear radiation has greatest biological effect in areas of the
body that have rapidly dividing cells. That is why, for example, radiation
attacks hair follicles and skin. Because cancer also involves rapid cell
growth, nuclear radiation is used to control and, in some cases, stop the
growth. It is ironic, but understandable, that radiation has the ability
both to induce and to cure cancer.
TABLE 3:
X-ray and gamma ray doses required to produce various somatic effects.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Radiation Standards
From our knowledge that risks
are involved in exposure to nuclear radiations and that a certain amount
of radiation will be released to the environment in the routine operation
of a nuclear reactor, the question arises, "Who will decide the maximum
allowable exposure for the general public?" In the United States, this
job is given to the Environmental Protection Agency (EPA). It is
the function of the Nuclear Regulatory Commission (NRC) to set emission
standards consistent with the guidelines of the EPA. These standards have
changed throughout the years but since the mid-1950s the maximum allowable
average exposure for the general population has been set at 170
mrem/year above background- This figure is based on the recommendation
that a person should receive no more than 5 rems of radiation above background
in a 30-year period. The recommendation is based on studies of the genetic
effects of radiation by a committee established by the United States National
Academy of Science-National Research Council and the United Kingdom Medical
Research Council in the mid 1950s. If we add this value of 170 mrem/year
to that of the approximate 190 mrem/year that a person normally receives
from natural and manufactured sources, we find that his or her total could
amount to about 360 mrem/year.
|
FIGURE 2: The induction of cancer by nuclear radiation is proportional to the dose received for doses greater than about 100 millirads. Experimental information is lacking for smaller doses. The graph illustrates three possible ways of extrapolating the cancer incidence-radiation behavior for low-dose exposures. |
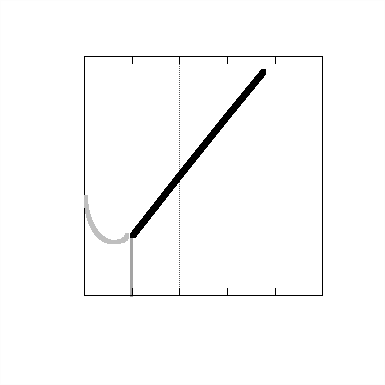 |
The 170 mrem/year exposure for the general population is controversial. That radiation induces human cell damage and forms of cancer such as leukemia is unquestioned. In fact, for exposures above 100 rads, experimental evidence indicates a direct linear relationship between incidences of cancer and radiation exposure (Fig. 2). Reliable data for exposures below 100 rads is lacking. Data for these low exposure levels are of extreme importance because they apply to levels of radiation in the realm of background exposures and those which could be achieved under present EPA guidelines. Any estimate of cancer incidences at low dosages must be based on an extrapolation of this curve. The feeling is that if the incidence rate increased as the exposure decreased, then it would be observed; it has not been. There are two other alternatives commonly offered. The first is that no effect occurs until some level of exposure, say around 100 millirad, is obtained. Then the cancer incidence rate increases with exposure as observed. This is referred to as the threshold hypothesis. If this were true, it would be the best alternative. The second proposal is that the observed linear relationship continues down to zero exposure. This is the linear hypothesis. In the absence of conclusive contrary evidence, the linear hypothesis is the most conservative assumption. Based on this assumption, it has been estimated that an added exposure of 170 mrem per year to the entire population for 30 years would produce 16,000 cancer cases annually. These estimates are probably realistic if the entire population did, in fact, get 170 mrem/year. However, the law requires that at the site of any nuclear power plant the average annual exposure to an individual cannot exceed 5 mrem. In fact, annual exposures during routine operation are much less than 5 mrem. Thus public concerns for radiation exposure focus more on accidental releases of large amounts of radioactivity rather than routine releases.
4National Academy of Sciences Committee on Biological Effects of Ionizing Radiation (BIER V) in 1990 confirms the linear model for solid tumors, but finds a linear-quadratic behavior for leukemia (Science 247 (1990) 23-23).
| This document was last modifed on Monday, 13-Nov-2000 18:29:58 EST
and has been accessed [an error occurred while processing this directive] times.
Address comments and questions to:
|